Neoadjuvant Therapy: Overview
“Neoadjuvant” therapy refers to any treatment that is given for cancer before the main treatment, with the goal of making the main treatment more likely to be successful. As surgery is the main treatment for breast cancer, neoadjuvant therapy for breast cancer is any treatment administered prior to surgery. It is usually in the form of systemic (drug) therapy, but can on occasions be local therapy (radiation).
Background:
Early stage breast cancer treatment involves a combination of local and systemic therapies, which can be delivered in a variety of possible sequences.
Historically, the treatment of breast cancer involved aggressive surgical resection, an approach based on the rationale that the more complete the removal of tissue, the less likely the disease would be to recur. However, it became apparent that even after radical mastectomy breast cancer could still recur.
Over the past half century, breast cancer management has evolved from primarily surgical therapy, to a multidisciplinary approach including surgery, radiation therapy and systemic therapy. This shift is based on an increased understanding of invasive breast malignancy as a “systemic” disease, and is based on the improved outcomes with the addition of adjuvant systemic therapy to local regional therapy.

Systemic therapy refers to breast cancer treatment that targets the entire body, and when given for non-metastatic invasive breast cancer is primarily intended to reduce the risk of distant recurrence. The evolution of systemic therapy for breast cancer began in the early 1970s with the first studies assessing the use of chemotherapy after resection.
Adjuvant systemic therapy can reduce recurrence and improve survival rates by killing cells that may have escaped the primary tumour bed via lymphatics and blood vessels. This has led researchers to hypothesize that giving systemic therapy before surgery (neoadjuvant) might improve outcomes because it would destroy undetectable microscopic circulating cells.
There are two main types of breast cancer treatment: Local and Systemic.
LOCAL TREATMENTS: Treat cancer cells in the breast area only
Surgery and radiotherapy are “local” treatment modalities.
SURGERY
Surgery involves removing any visible cancer in the breast and/or axilla.

RADIOTHERAPY
Radiotherapy kills cancer cells in the area it is aimed at, and is similar to the x-rays that are used when you have a chest X-ray.
SYSTEMIC TREATMENTS: Using drugs that can reach all parts of the body
Systemic therapies are drugs which spread throughout the body to treat any microscopic cancer cells, wherever they may be, and include chemotherapy, hormonal therapy, targeted drugs, and immunotherapy.
CHEMOTHERAPY
Chemotherapy is a cytotoxic treatment that is given to kill cancer cells throughout the body. For breast cancer, it is usually given intravenously (though a drip or injection into the vein) every 1-3 weeks, for a total of 12-24 weeks.
TARGETED THERAPY
Targeted therapy, such as trastuzumab (Herceptin), is given to women with HER2 positive breast cancer. This type of treatment works by targeting the HER2 receptors on tumour cells, stopping the cells from dividing and growing. They are usually given intravenously (through an IV drip) once every three weeks for a year in total, including any trastuzumab that you might receive before surgery. Other HER2-targeted drugs such as pertuzumab (Perjeta) may also be used.
HORMONAL THERAPY
Hormonal (endocrine) therapy is a tablet that is taken every day, for women with hormone receptor positive (HR+) breast cancer (oestrogen (ER) and/or progesterone (PR) positive). For premenopausal women, a medication such as Zoladex may be also used to stop the ovaries from making oestrogen, or they may be removed surgically. Hormonal therapy works by interfering with the signal that oestrogen sends to cause this type of breast cancer to grow. Hormonal treatments are usually given for 5 years or longer.
What is Neoadjuvant Chemotherapy (NAC)?
In most cases of breast cancers, the primary, definitive treatment is surgery to remove the cancer. Additional systemic treatment, such as chemotherapy or hormone therapy, is used either before or after the primary therapy. Extra treatment in addition to surgery, given after primary therapy is referred to as adjuvant therapy (postop) whereas extra treatment given before primary therapy is referred to as neoadjuvant therapy (preop).
Systemic therapy given for non-metastatic invasive breast cancer whether administered pre or post op, is intended primarily to reduce the risk of distant recurrence. The purpose of administering it prior to surgery, is also for the treatment of locoregional disease.
- Neoadjuvant therapy may locally “downstage” the tumour, which may allow less extensive surgery to the breast and/or axilla, potentially allowing breast conserving surgery in circumstances where mastectomy was initially required, leading to improved cosmetic outcomes, and potentially avoiding axillary node clearance, thereby reducing the risk of postoperative lymphoedema.
- Neoadjuvant chemotherapy clears axillary nodal disease in approximately 35% of patients overall. Patients with triple-negative cancer will have their nodes cleared in approximately 50% of cases and patients with ER-positive disease in less than 10%. With a combination of chemotherapy and trastuzumab, nodes are converted from involved to clear in up to 70% of HER2+ cancers.

- Neoadjuvant therapy also permits an early evaluation of the effectiveness of systemic therapy. The surrogate endpoint, the presence and extent, or absence of residual invasive cancer after neoadjuvant chemotherapy (NAC), is a strong prognostic factor for subsequent recurrence, especially in triple-negative breast cancer (TNBC) and human epidermal growth factor receptor 2-positive (HER2+) breast cancer.

Neoadjuvant chemotherapy was originally employed in breast cancer patients who presented with “inoperable” disease in order to render them operable candidates. More recently however, potential benefits of NAC have been increasingly appreciated in early operable breast cancer.
While surgery remains the first treatment recommended for the majority of patients with early breast cancer, neoadjuvant chemotherapy is being used with increasing frequency in the multidisciplinary treatment of patients with operable breast cancer.
Large clinical trials have shown no difference between the same systemic therapy given pre- or post-surgery on disease free and overall survival. Early randomized controlled trials comparing NAC with adjuvant chemotherapy (AC) in breast cancer patients, including the National Surgical Adjuvant Breast and Bowel Project B-18 and B-27 did not demonstrate the expected survival advantage from early treatment of micrometastatic disease. Instead, AC and NAC were shown to have equal efficacy in improving overall survival. The survival analysis from these older trials may now be less clinically relevant because of advances in chemotherapy regimens and increased knowledge regarding the molecular subtypes of breast cancer, and NAC may be more likely to benefit patients with rapidly growing and early spreading subtypes of breast cancer, but a survival advantage for patients with selected subtypes is, as yet, unproven.

Neooadjuvant therapy may have several advantages. By downstaging of the tumour, chemotherapy can convert patients who are candidates for mastectomy to breast-conserving surgery candidates. Furthermore, it has potential to reduce excision volumes in patients with large cancers who are already candidates for breast conserving surgery, thereby improving cosmetic outcomes. Another surgical advantage is downstaging of the axilla so that lymph node dissection can be avoided in selected patients reducing surgical morbidity.
Neoadjuvant therapy also allows to monitor response to therapy at an early stage; potentially allowing time and flexibility to switch therapies if patients do not respond.
All early stage breast cancer patients identified as likely to require adjuvant chemotherapy should be considered for neoadjuvant therapy, as they may potentially benefit from treatment before surgery. Factors favouring neoadjuvant therapy in patients with operable breast cancer include: lymph node-positive disease; high tumour volume-to-breast ratio; specific biological features of primary cancer (high grade, hormone receptor-negative, HER2-positive, triple negative cancer); younger age. Patients with HER2-positive and triple negative cancers have the highest probability of achieving pathological complete response after neoadjuvant therapy making them good candidates for consideration
One of the benefits of initiating neoadjuvant therapy is expedited treatment of both in-breast disease and micrometastatic disease.
Treatment Sequencing
The traditional treatment paradigm of surgery followed by chemotherapy followed by radiotherapy is increasingly altering. Different sequencing allows treatment to be individualised, tailoring it to the tumour biology and the patient’s unique situation and preferences.

Research has classified breast cancer into five intrinsic subtypes on the basis of molecular testing and this can help guide treatment. Each type is associated with a particular phenotype and has a distinct behaviour, risk factors and sensitivity to systemic therapy that help in the selection of the appropriate regimens and medications. See Initial Treatment Decisions

The efficacy of neoadjuvant treatment is assessed by evaluating the clinical and radiological response during therapy, and the pathological response after surgery. The likelihood of achieving a significant response is predicted by cancer phenotype; patients with HER2-positive and TNBC have the highest probability of achieving pCR after NAC. In triple negative cancers (TNBC), pCR rates up to 64% can be achieved. The highest rates have been described for ER-negative/HER2-positive tumours (up to 76%) .
Although there is consensus on the patient subgroups most likely to benefit from neoadjuvant treatment, its utilisation in clinical practice remains highly variable. All early stage breast cancer patients identified as likely to require adjuvant chemotherapy should be considered for NAC, as they may potentially benefit from treatment before surgery.
Factors favouring NAC in patients with operable breast cancer include:
- High tumour volume-to-breast ratio
- Lymph node-positive disease
- Biological features of primary cancer (high grade, hormone receptor-negative, HER2-positive, TNBC)
- Younger age
Pathologic Complete Response (pCR)
Trials have shown similar survival for adjuvant versus NAC, however, a major advantage of NAC is that it has prognostic significance. In some cases, neoadjuvant therapy will shrink the cancer so much that the pathologist can’t find any residual cancer in the operative specimen. This is called a pathologic complete response (pCR), and survival is significantly improved when compared with a patient who does not achieve a pCR. If a pCR is not achieved, there is an opportunity to give additional chemotherapy after surgery.
Although a pCR is encouraging, it doesn’t mean the cancer will never return. And, many people who don’t have a pCR will still do very well. It is essential to communicate to patients that achieving pCR is not the only positive outcome measure of NAC, as downstaging from mastectomy to BCS, irrespective of pCR, is itself a positive outcome. Complete pathological response has been shown to be a powerful predictor for good long-term outcome in ER-negative but not ER-positive cancers.
The likelihood of a pCR is highest for tumours that are triple negative or are HER2+. When these features are seen on a diagnostic core biopsy, especially in a younger woman, NAC is likely to be considered. In contemporary clinical practice patients with stage II or III HER2 positive breast cancer are likely to be offered chemotherapy and trastuzumab and pertuzumab in the neoadjuvant setting.
Other advantages of NAC are that tumour shrinkage reduces the need for mastectomy and for axillary lymph node dissection, allows time for genetic testing and surgical planning in the setting of a strong family history and allows more options for breast reconstruction when mastectomy is required.
Neoadjuvant chemotherapy may be recommended:
- If you have inflammatory breast cancer
- If you have inoperable “locally advanced” breast cancer (LABC) involving skin
- To reduce the size of the tumour so that you can have breast conserving surgery (lumpectomy) instead of mastectomy
- To reduce the size of the tumour so that a smaller amount of tissue can be removed – this may give you a better cosmetic outcome
- To give you time to have genetic testing if you have a strong family history of breast cancer – you may decide to have a different type of surgery if you are found to have an inherited breast cancer gene mutation
- To delay surgery if you are pregnant so that you can deliver your baby as near to full term as possible (certain breast cancer chemotherapy drugs have been found to be safe in pregnancy)
- To give you time to consider your surgical options, including breast reconstruction.
Tumour Response and Surgical Planning
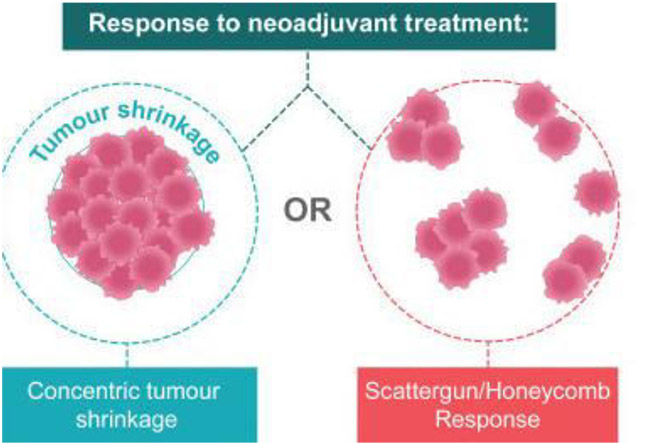
- Tumours shrink in different ways after neoadjuvant therapy. The response to neoadjuvant chemotherapy may be complete (CR), partial
but concentric or partial and honeycomb-like. In the latter case breast conservation may not be feasible.
- Patterns of tumour response may influence surgical planning. There are two general patterns of response after NAC: concentric shrinkage or a scattergun/honeycomb response, where the residual carcinoma presents as multiple, scattered foci over an ill-defined tumour bed. This latter pattern of response is particularly problematic when planning surgical procedures, as obtaining clear margins is more difficult.
Key points
- There is no survival advantage to neoadjuvant systemic therapy as compared to adjuvant systemic therapy. Neoadjuvant chemotherapy (NAC) does not prolong survival compared with adjuvant chemotherapy, but reduces the need for mastectomy and axillary lymph-node dissection, and thus surgical morbidity, without increasing the risk of locoregional recurrence
- Neoadjuvant systemic therapy allows for assessment of tumour response to therapy and pathological complete response is associated with improved survival outcome.
- When a decision is made to administer neoadjuvant chemotherapy, current consensus reports support the use of all planned chemotherapy before surgery.
- There is a varying response to chemotherapy by molecular tumour subtypes; the highest rates of pCR are seen in triple-negative and HER2-positive disease. Patients with high-grade hormone receptor (HR) negative and/or HER2-positive breast cancers are more likely to experience pathological complete response to NAC than those with low-grade, HR+ tumours.

- Rates of eligible patients for breast-conserving surgery are increased after neoadjuvant systemic therapy without compromising local regional control.
- Lumpectomy, following NAC, does not need to remove the entire volume of breast tissue initially occupied by the tumour.
- Sentinel lymph-node biopsy (SLNB) after NAC, accurately stages the axilla and is associated with a low rate of nodal recurrence in patients presenting with clinically negative axillary lymph nodes
- In patients who convert to clinically node-negative disease, SLNB after NAC has a false-negative rate of <10% when ≥3 sentinel nodes are identified. Axillary staging with sentinel node biopsy including excision of the biopsy-proven nodal metastasis at diagnosis after apparent response to neoadjuvant chemotherapy in initially N1 disease is feasible and has a low false-negative rate in selected patients.
- The relative contribution of pre-NAC and post-NAC stage (degree of pathological response) to local control is uncertain; and tailoring radiotherapy based on response to NAC is being evaluated in ongoing trials
- The neoadjuvant approach has also become an important vehicle for the evaluation of new agents
- Future directions include identification of exceptional responders to neoadjuvant systemic therapy who may not require surgical intervention

Neoadjuvant Therapy: Patient Information:
Neoadjuvant Patient Decision Aid
Neoadjuvant Therapy: Book Chapters
Neoadjuvant Therapy: Book Chapters-1