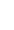Neoadjuvant Therapy: Personal Approach and Practical Aspects
Neoadjuvant Therapy Personal Approach and Practical Aspects
Neoadjuvant treatment offers a number of benefits for patients with early breast cancer, and is an important option for consideration by multidisciplinary teams. As the breast surgeon is almost always the first specialist with whom the patient with a new breast cancer diagnosis has contact, it is incumbent on the breast surgeon to consider the option of neoadjuvant therapy.
In my opinion, all patients should be discussed in a multidisciplinary meeting (MDM) at the time of diagnosis prior to any treatment plan being instigated so that they may be offered the full range of treatment sequencing options.
Breast cancer treatment has evolved into a truly multidisciplinary endeavour, requiring a team that includes breast surgeons, medical and radiation oncologists, radiologists, pathologists, geneticists, plastic surgeons and nurses in patient care.

Above: The Breast Centre Weekly Multidisciplinary Meeting. Pictured clockwise from top right: Breast surgeon, medical oncologist, radiation oncologist, MRI radiologist, pathologist and breast radiologist,
When discussing neoadjuvant treatment, it is crucial that provisional histological grade (i.e. derived from the core biopsy specimen), hormone receptor and HER2 result, including SISH if appropriate, and radiological results are available at the MDM, and discussion should include the proposed surgical plan after treatment, which will be determined by the tumour response to NAC and also the radiotherapy plan.

Despite literature showing its efficacy, the use of neoadjuvant therapy varies widely. NAC is used in only 3.8% of cases in the United States and in 4.43% of operable breast cancer cases in Australia (1) and New Zealand compared with 14% in Jane O’Brien’s practice in 2019.
A retrospective analysis of prospectively collected data from the BreastSurgANZ Quality Audit (BQA) database identified patients registered with early breast cancer who received NAC from 2011 to 2016. A total of 55,757 cases of breast cancer were identified from 2011 to 2016, of which 2,469 (4.43%) cases underwent NAC. The proportion of patients receiving NAC increased from 3.08% in 2011 to 6.65% in 2016.
The results show that the proportion of patients receiving NAC in early and locally advanced breast cancer is slowly but gradually increasing. In 2015, Read et al. reported that less than 3% of women with operable cancer in Australia received NAC. (2) In the light of the evidence demonstrating the benefits of NAC, it is encouraging to see that this trend is increasing in Australia although it still falls short of the estimated 20% of patients with breast cancer that might benefit from NAC .
Evaluation of the American National Cancer Database from 2010–2015, demonstrated that approximately 20% of patients who received chemotherapy, received it upfront in the neoadjuvant setting, (cf Jane O’Brien -30% in 2018) with the proportion significantly increasing in the USA from 15.7 to 26.0% between 2010-2015.
Neoadjuvant Therapy Algorithm
I find a helpful way of outlining the possible treatment pathways is with the use of a treatment algorithm, which outlines the decision making process in flow chart format.
Practices change over time, and I have developed my own Neoadjuvant Therapy Algorithm, based on my current practice and the way I currently work through the decision making in each individual patient.
Neoadjuvant therapy has long been recommended as the optimal initial treatment for “inoperable” locally advanced breast cancer (LABC), such as inflammatory breast cancer (IBC) in order to render the disease operable.
In addition, all early stage breast cancer patients identified as highly likely to require adjuvant chemotherapy should be considered for neoadjuvant therapy, as they may potentially benefit from treatment before surgery.
For early HER2+ or TNBC which are small (T1-<2cm) and apparently node negative (N0) at the time of diagnosis, although it is highly likely that chemotherapy will be recommended at some point, there may not be enough information available with which to select the most appropriate chemotherapy regimen prior to surgery. As such, initial surgery is usually undertaken, followed then by the appropriate adjuvant systemic therapy, based on the operative pathology.

For HER2+ or TNBC which are either larger (T2 or T3->2cm) or pathologically confirmed to be node positive on preoperative needle biopsy (N1), the medical oncologist has enough information available on which to base their decision making regarding the appropriate systemic therapy regimen, and as such preoperative or neoadjuvant therapy is an appropriate option to consider.
The potential benefits of preoperative treatment in this situation are:
- One of the benefits of neoadjuvant therapy is the expedited treatment of both in-breast disease and systemic micrometastatic disease.
Neoadjuvant therapy is able to be commenced without delay, thereby initiating the treatment of potentially undetectable microscopic circulating cells almost immediately, rather than many weeks later. Adjuvant systemic therapy is not routinely commenced for at least 4 weeks post operatively. Allowing for a week or fortnight at least between diagnosis and surgery, and often longer in the setting of more major surgery such as mastectomy, either unilateral or bilateral, in conjunction with complex immediate reconstruction, (which inevitably takes longer to arrange and schedule), means that drug therapy addressing whole body treatment may not be commenced for many weeks following the initial diagnosis.
- In the unfortunate event of a post-operative wound complication, the commencement of post-operative (adjuvant) chemotherapy may need to be delayed longer than the usual 4 weeks, until wound healing is complete. Delaying oncologically important systemic therapy is not ideal Undergoing systemic treatment prior to surgery avoids this delay, and is therefore particularly appropriate in those undergoing more major surgery, where the risk of a wound complication of some type is inevitably higher, and also in patients assessed to be at a higher than average risk of wound problems such as the obese, diabetics and smokers.
- For patients with significant comorbidities, an oncology prehabilitation programme can be undertaken during neoadjuvant therapy, in order to optimise conditioning prior to surgery. These programmes address. amongst other things, exercise, diabetic and weight control and smoking cessation. Patients initially assessed as being medically unsuitable for immediate reconstruction at the time of diagnosis because of smoking or severe obesity (eg BMI>35), may potentially be deemed eligible if comorbidities are successfully addressed during the six month course of neoadjuvant therapy.
- Neoadjuvant therapy may lead to the local downstaging of disease in the breast and/or axilla, potentially allowing less radical surgery if desired.

- For the patient who chooses mastectomy, or in whom mastectomy is the only appropriate surgical option, undergoing neoadjuvant therapy, which is usually administered over a period of six months, provides the patient with ample time in which to carefully consider and make surgical treatment decisions, and to explore reconstructive options if so desired.
- The turnaround time for genetic testing results, even when fast tracked, is a matter of weeks, and results are therefore not available to the patient undergoing initial surgery prior to having to make decisions about the nature of their surgery. In patients undergoing neoadjuvant therapy, genetic testing results become available prior to surgical decision making, enabling the patient to modify their treatment plans if appropriate. For example, a patient with a 4-5cm unifocal TNBC, who may potentially be suitable for breast conserving surgery if neoadjuvant therapy leads to significant shrinkage of the tumour, may, if genetic testing identifies a BRCA mutation, elect instead to undergo bilateral mastectomy +/- immediate reconstruction, rather than lumpectomy.
- Although it happens relatively infrequently in practice, as neoadjuvant therapy allows for the “real time” evaluation of response to treatment, there is therefore an opportunity to switch therapeutic agents if patients do not respond.

- The pathological response to neoadjuvant therapy as adjudged by the presence and extent, or absence of residual invasive cancer after treatment, is a strong prognostic factor for subsequent recurrence. The patient who achieves a pathological complete response (PCR) can therefore be informed that their prognosis is likely to be excellent, irrespective of their disease stage at the time of diagnosis.
- The patient who does not achieve a PCR, is eligible for further treatment with different, often newer agents following surgery, and in HER2+ and TNBC, there is randomised controlled trial evidence that this “adjuvant” therapy, administered following surgery, improves outcomes in those in whom the “neoadjuvant” therapy, given prior to surgery, does not achieve a complete pathological response. In this situation, the sequencing of treatment thereby renders the patient eligible to additional, potentially beneficial therapy, to which they would otherwise not have been exposed.
- There are specific clinical situations in which neoadjuvant therapy may provide perceived benefits with respect to timing. For example during pregnancy, it is safe to administer chemotherapy after the first trimester, but ideally it is best avoided around the time of delivery because of the repurcussions of low blood counts, and so it may therefore be preferable to administer neoadjuvant chemotherapy followed by surgery, rather than the reverse sequence, in thi situation
- Currently postoperative radiotherapy recommendations are usually based on the stage of disease at diagnosis. For example, a patient pathologically confirmed to be node positive at the time of diagnosis, who undergoes mastectomy and axillary surgery following neoadjuvant therapy, is currently highly likely to be recommended post mastectomy radiotherapy (PMRT), even if they are converted to being node negative by neoadjuvant therapy based on operative pathology. These patients who achieve a nodal PCR, are felt to be at a low risk of locoregional recurrence, and there are currently trials being undertaken to assess the safety of potentially “de-escalating” post operative radiation based on the pathological response to neoadjuvant therapy. If such trials were to confirm the safety of omitting radiation in this circumstance, this would provide yet another compelling argument to consider neoadjuvant therapy.
There are some potential disadvantages of neoadjuvant therapy:
- It is very time intensive for both patients and specialists, involving more consultations in total.
- It also involves more interventions. Marker clips are routinely inserted into the breast tumour +/- if appropriate, involved axillary node, prior to the commencement of neoadjuvant therapy, to allow localisation prior to surgery in the event of a complete clinical and radiological response to therapy.

- More imaging is usually undertaken, with check ultrasound +/- mammogram, +/- MRI partway through neoadjuvant therapy to assess response to treatment and for surgical planning purposes, particularly in patients in whom the aim is for local downstaging of the breast tumour in order to facilitate breast conservation.
- Some medical oncologists may on occasions modify their preferred chemotherapy regimen based on genetic testing results, which as outlined above, are not available for a matter of weeks after diagnosis, certainly not at the time neoadjuvant therapy is commenced.
- One of the big advantages of neoadjuvant therapy is that it can be commenced immediately. This can however potentially compromise fertility interventions in premenopausal women. In the situation of primary surgery, fertility interventions such cycle stimulation, egg collection, IVF or the harvesting of ovarian tissue. In women undergoing initial surgery, these interventions are routinely undertaken during the 4 week period between surgery and the commencement of adjuvant chemotherapy.
In my practice consideration of NAC starts at the time of first consultation.
- Pt with confirmed diagnosis- T2 or N1 and favourable subtype (HER2+ or TNBC)

- Start preliminary discussion with patient regarding the potential sequencing of treatment

Diagnostic Work Up:
- Bilateral Mammography/Ultrasound
- Core Bx- with hormone receptors and HER2, Incl SISH
- +/- Breast MRI
- Targeted scan ipsilateral axilla/SCF +/- image guided nodal biopsy
- ? Pretreatment SLN only rarely considered- if histol is critical to treatment decision making
- Staging- CT/bone scan +/-PET scan
- Bloods-including tumour markers and ? including genetic testing
Treatment Planning:
- MDM discussion
- Medical Oncology Consultation
- Consider Radiation Oncology consult
- Placement marker clips in breast tumour +/- axillary node
- Fertility specialist if appropriate
- ? Plastic Surgeon
Treatment Monitoring:
Disease progression occurs in only 3%
- Book review for 4-8 weeks. Ie after 2/3 cycles chemo (AC)
- Genetic tests may be avail
- ? Plastic surgeon
- Book further 8 week review , often with imaging prior ie after 2 cycles weekly Taxol at which time there is 10 weeks treatment remaining- often able to confirm tentative surgical options at this time.
- ? Plastic surgeon
Surgical Planning:
Nature of Surgery Based on:
- Extent of disease at presentation
- Patient choice
- Clinical /Imaging Response to NAC
- Genetic testing results if performed

- Book 8 week review ie 2 weeks prior to completion chemo
- At that appointment book operation date -4 weeks post completion chemotherapy.
- Provide patient with request slip for bloods checked 1 week preop

Post Op
- Determine need to consider additional “adjuvant” treatment if residual disease present on operative pathology
- Determine need for/extent radiation